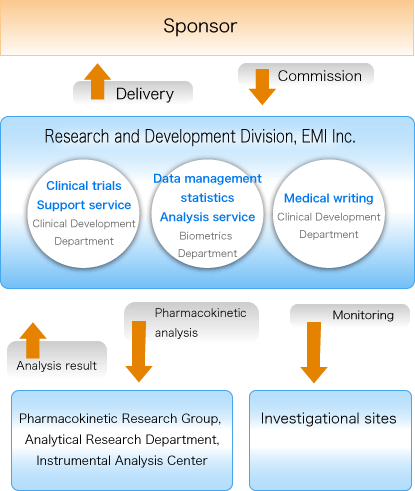
Our Services
Drug Research
Pharmacokinetic test (Pharmacokinetic Research Group, Analytical Research Department)
- Measurement of drug concentration levels
- Measurement of metabolites
- Biodegradability
Formula quality test (Formula Study Group, Analytical Research Department)
Formulation stability test
- Stability test on unpackaged products (temperature, humidity, and light)
- Stability of pulverized products (temperature, humidity, and light)
Incompatibility test with concomitant drugs
- Stability test after dissolution
- pH-change test
- Incompatibility test under diffused indoor light
Clinical Development
Clinical development
- Preliminary evaluations of investigational sites
- Preliminary evaluations of investigators
- Development of clinical trial protocol (draft), case report form sample (draft), and informed consent form (draft)
- Request to investigational sites for a clinical trial and arrangement for contract signing
- Preparation of documents to be submitted to institutional review boards (IRBs).
- Dispensation and collection of study drugs
Tasks related to monitoring
- Development of monitoring report
- Confirmation of compliance with protocol, Good Clinical Practice (GCP), and related items
- Response to an adverse event
- Acquisition of essential documents from investigational sites
- Validation of case report forms referring to source documents
- Collection of case report forms
- Administration for closing a clinical trial
- Inspection and quality control of case report forms
Data management/statistical analysis (Biometrics Department)
- Database design
- Data input and fixed services
- Development of statistical analysis plan
- Development of statistical analysis report
- Development of clinical trial data lists and graphs
Medical writing (Clinical Development Department)
- Development of clinical study report (draft)
- Development of application outline (draft)
Pharmaceutical Consultation
Consulting on drug pharmaceutical application
- Support for drug pharmaceutical application
- Development of marketing authorization application form (including partial changes)
- Development of marketing license application form (including application for renewal)
- Development of manufacturing license application form (including application for renewal)
- Development of distribution license application form (including application for renewal)
- Development of application form for registration of the Drug Master File (including application for amendment)
- Development of application form for accreditation of foreign manufacturer (including application for renewal)
- Domestic administrator upon registration of Drug Master File
- Substitute for application and other procedures for a foreign manufacturer
Support for tasks related to Good Manufacturing Practice (GMP), Good Quality Practice (GQP) and Good Vigilance Practice (GVP)
Consultation on other general issues related to pharmaceuticals
Operational arrangement for clinical trials