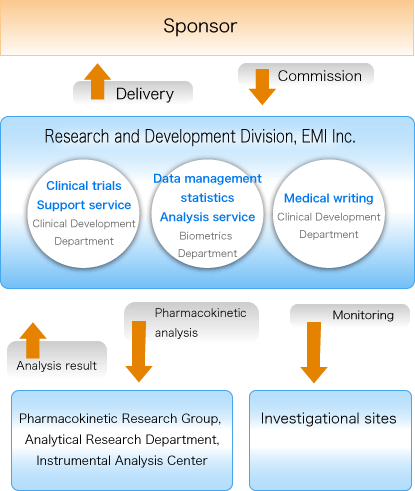
Clinical Development
Clinical Development
Clinical development
- Preliminary evaluations of investigational sites
- Preliminary evaluations of investigators
- Development of clinical trial protocol (draft), case report form sample (draft), and informed consent form (draft)
- Request to investigational sites for a clinical trial and arrangement for contract signing
- Preparation of documents to be submitted to institutional review boards (IRBs).
- Dispensation and collection of study drugs
Tasks related to monitoring
- Development of monitoring report
- Confirmation of compliance with protocol, Good Clinical Practice (GCP), and related items
- Response to an adverse event
- Acquisition of essential documents from investigational sites
- Validation of case report forms referring to source documents
- Collection of case report forms
- Administration for closing a clinical trial
- Inspection and quality control of case report forms
Data management/statistical analysis (Biometrics Department)
- Database design
- Data input and fixed services
- Development of statistical analysis plan
- Development of statistical analysis report
- Development of clinical trial data lists and graphs
Medical writing (Clinical Development Department)
- Development of clinical study report (draft)
- Development of application outline (draft)
Operational arrangement for clinical trials